I heard a little more recently on the contaminated blood scandal of the 1980s, where huge numbers of people with hemophilia were given treatments for their condition that were contaminated with viruses, including HIV and Hepatitis C.
Early on, there was little knowledge about the true risks of these viruses, and no proper testing available (certainly not widely available), but it turns out that some of this story is even worse than simply being given contaminated blood products.
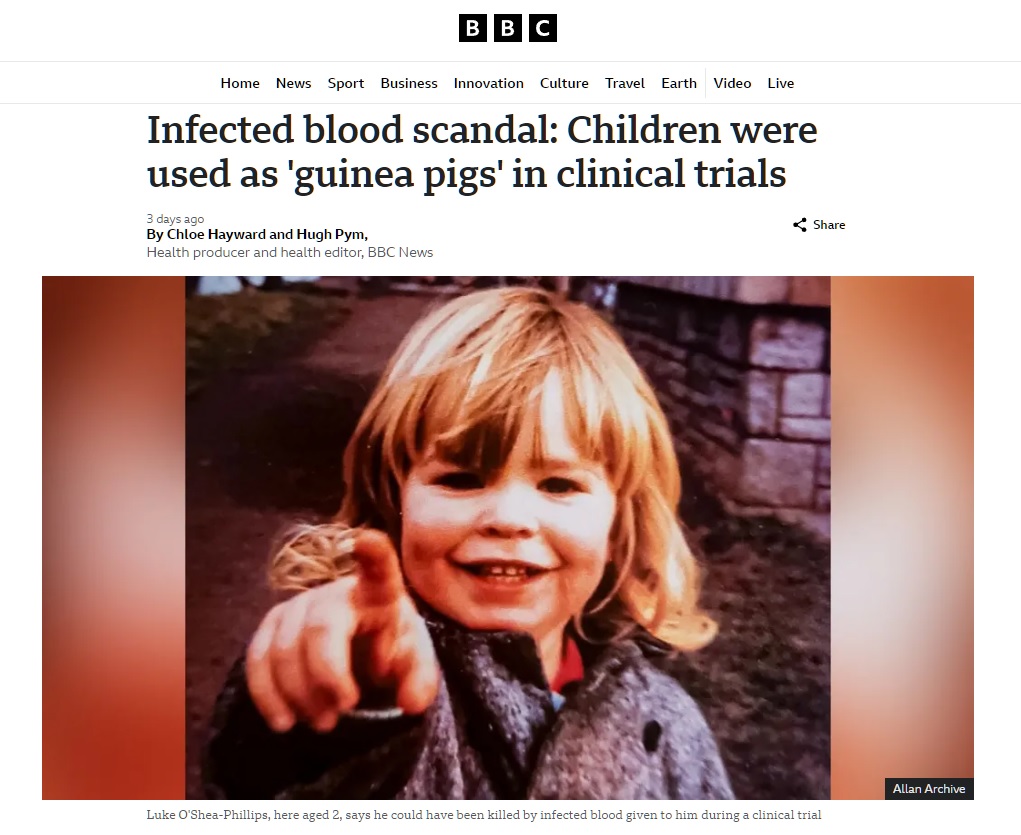
An inquiry in the UK is currently focused on a specific series of infections that occurred at one particular school, which had a hemophilia treatment center on-site and which, it turned out, was also conducting research into novel treatments for the disease. At a superficial level the research seemed sound – it was asking the question: would a new heat-treated version of the clotting factors needed to treat hemophilia be safer than the standard treatments? Unfortunately, the heat-treatment wasn’t sufficient to completely inactivate the viruses – but the story is much worse than just that.
Several people have come forward who not only were infected with the viruses anyway, but possibly didn’t need treatment for their hemophilia at the time they were given the experimental treatments and, worse, neither they nor their parents were properly consented for the research. When the true risks of infection were obvious, subjects weren’t told, and some weren’t even informed of their infections for a year or more. One doctor involved in the study, a Dr Samual Machin (now deceased) is quoted during the inquiry:
“This would have been discussed with his mother, although I acknowledge that standards of consent in the 1980’s was quite different to what it is now,”
At the time, the subjects were all children, and several of the parents denied being properly told about the research, and that they would not have consented had they been told. Further documents showed that untreated patients were highly sought after, and that trying out the new heat-treated therapy may have been prioritized over the patients actual medical needs.
There are several core principles of research at stake here – beneficence (doing good), non-maleficence (do not do anything bad) and autonomy (being able to make your own decisions). Even a cursory look over the stories of this case show that none of those principles were upheld in this research, and of course the fact that the heat-treatment didn’t work to inactivate the viruses makes it all the worse (arguably if the research was a stunning success there wouldn’t be an inquiry into any of this…but that’s a whole other topic for discussion).
While there are regulatory rules for ensuring proper scientific conduct of research, there are also ethical rules. The two have some overlap, but they really are distinct frameworks, within which researchers have to function. The sad fact is that many of the rules and protections that we now take for granted were imposed as a direct consequence of past unethical human experimentation (which leads to another discussion about how what is considered “ethical” changes over time). From the perspective of human subjects and safety in particular, these are reflected primarily in the document we know as the ICF – the Informed Consent Form.
While it’s true that many aspects of subject safety, rights, and welfare are contained within the research protocol, the protocol itself is a highly technical description of the research and as more of a scientific justification and research plan. The ICF on the other hand is intended to be seen and understood by the study subject themselves, and includes a discussion on the research protocol (visits, procedures, timeline, risks etc.) in addition to making them aware of certain other rights afforded to them, including the right to leave the study at any time and any other treatment options available. The research site’s institutional review board (IRB) would have reviewed and edited the ICF to ensure that it accurately reflected the research risks and benefits prior to being seen by a potential subject. IRBs include subject-matter experts as well as members of the lay public to ensure clarity and understanding. Not only are potential study participants supposed to be given the opportunity to ask questions before signing the ICF, but they receive a copy and a copy remains in their medical record.
The fact that no such process occurred in these contaminated blood product studies is obvious.
Unfortunately, stories like this from research done decades ago, and which clearly doesn’t meet current standards, color everybody’s opinion of medical research. I have had parents refuse to consent for a study because “The sponsor is [insert drug company with research scandal] and I don’t trust them,” even though the drug in the study had nothing to do with the risks from the older scandal. I heard about one parent who turned down a study because of a poorly written ICF, that ironically overstated the risks in a summary paragraph that implied the drug had never been given to anyone before – some people might have continued reading, but this parent did not (they did however provide that feedback to the investigator, so we were able to modify the ICF to make it more clear). One time I had a sponsor try to avoid explaining all the risks (for the control therapy, not the study drug) in the ICF and instead provide a patient hand-out or “your doctor will explain this to you” verbiage. Despite our warning that the IRB would reject this ICF, they insisted on submitting it and of course wasted a whole IRB review cycle as they were forced to revise and resubmit the document with our recommended wording. This wasn’t an intentional thought to mislead, they genuinely thought it was a more efficient process and would avoid scaring the subject unnecessarily, but we knew that the ICF should be a stand-alone record of the subject being truly informed before consenting.
One of my experiences with obtaining consent sticks out to me – it was a slowly-recruiting antibiotic study and I was hoping to not have another screen-failure at my site. I had found a possible subject and was discussing the study with his mother and my study nurse. The mother was asking careful questions, clearly a little nervous (her son was in hospital after all!) and I was thinking that she probably wasn’t going to sign him up when she suddenly said “You know what, you’re the doctor and you know best, I’ll do whatever you say.” Massive red flag to me, as an investigator. Research isn’t the same as medical care – if “I knew what was best” we wouldn’t be having a conversation about a clinical trial! Also, if something were to happen during the study and she had rescinded the decision to me, then she as a mother would feel far worse than if she had made the decision truly believing she had made the best call. So I called “Investigator fiat” and screen-failed them. Every set of inclusion/exclusion criteria includes a line about “any other condition which, in the opinion of the Investigator. would interfere with the conduct of the study” and at that point I’m not sure that she fully understands the risks and benefits.
While we might celebrate some of these stories as holding ourselves to a high standard, the sad truth is the current standards of oversight and training that we have in medical research are much better than they were in the past mostly because of how poor they were in the past. Personally, I find it shocking that some of this occurred within my lifetime, and I think it behooves us to be mindful every day about how we conduct our research, placing the rights and welfare of our participants first.